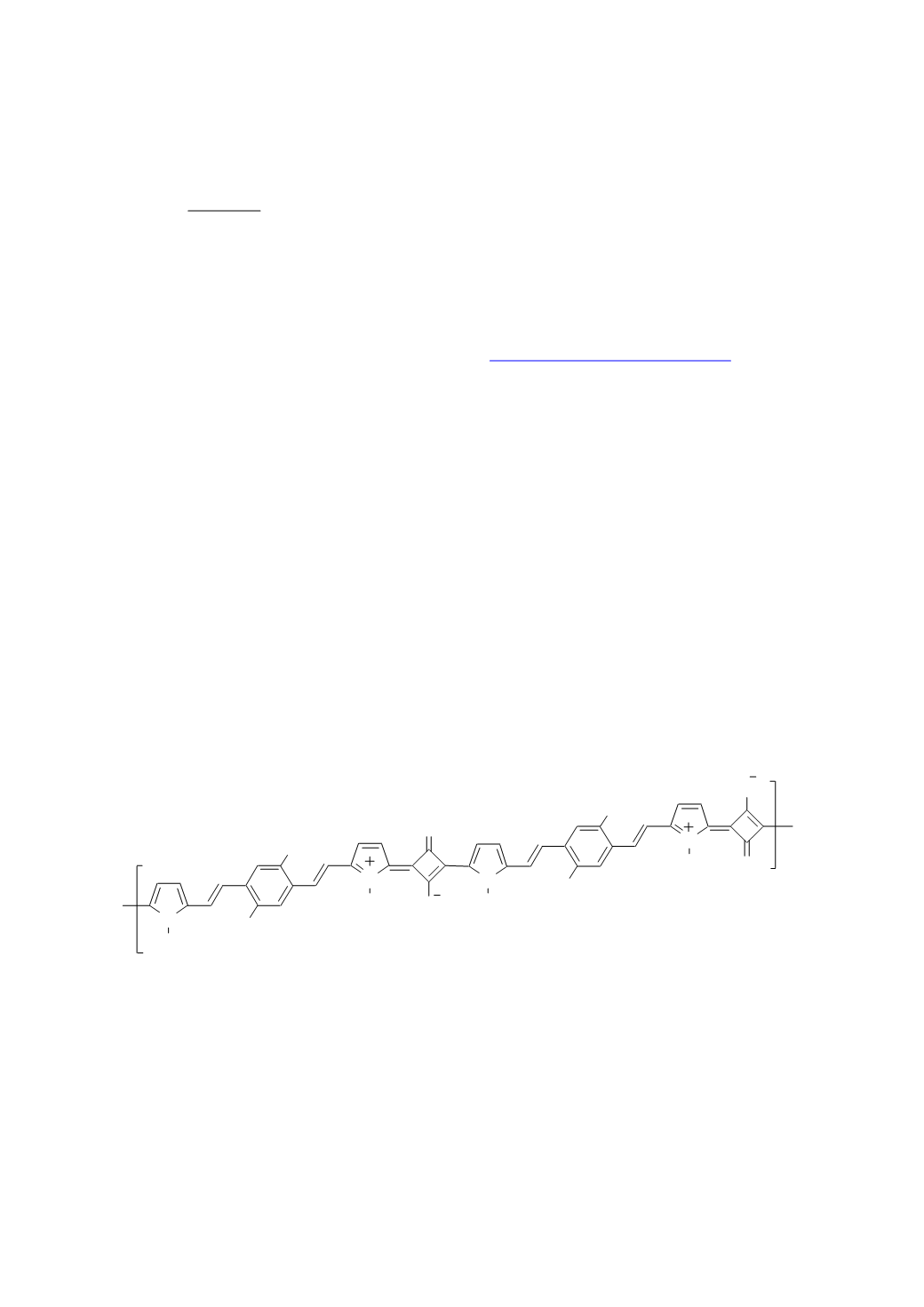
Squaraine-based polymers: towards optimized structures for efficient field-effect
transistors
A
. Broggi,
a
M. P. Bracciale,
a
M. L. Santarelli
a
, C. Kim,
b
A. Marrocchi
c
a
Sapienza University of Rome - Department of Chemical Engineering, Materials and
Environment, Via Eudossiana 18, Roma, 00184, Italy;
b
Department of Chemical and Biomolecular Engineering, Sogang University, Seoul 121-742,
Republic of Korea;
c
University of Perugia – Department of Chemistry, Biology and Biotechnology, Via Elce di
Sotto 8, Perugia, 06123, Italy; E-Mail:
Since their first isolation in 1959, squaraine compounds have gained an increasing important
role in modern materials science. Although they primarily played a role as sensitizers in
xerography, recently their most characteristic features (stability, strong and localized low
energy absorption, efficient emission, reversible redox behaviour, nonlinear optical
performances, ion sensing, wide molecular diversity) have been successfully exploited in
many different technologically relevant research areas including field-effect transistors [1].
From a structural point of view, squaraine-based compounds are the dicondensation product
of electron-rich molecules with squaric acid.
Here we report on the synthesis and characterization of a model
-conjugated polysquaraine
1
[2] (Figure 1) to be employed as semiconductor for efficient field-effect transistors
(OFETs). The design strategy in the present semiconductor aims at achieving high solubility
in common organic solvents and efficient charge transport. Enhanced solubility is achieved
by introducing alkoxy/alkyl chains on to the delocalized core peripheries. The squaric acid
core, being a four-membered tensioned ring, rigidifies the backbone, thereby planarizing the
structure and, ultimately, allowing the polysquaraine to arrange in a densely packed
arrangement.
Solution processable OFETs are preliminary fabricated for
1
, and the device response
characterized.
N
N
N
N
C
12
H
25
R
R
C
12
H
25
OC
8
H
17
C
8
H
17
O
OC
8
H
17
C
8
H
17
O
O
O
O
O
n/2
1
Figure 1
References
[1] L. Beverina, P. Salice,
Eur. J. Org. Chem.
2010
, 1207–1225.
[2] A. Presciutti, F. Asdrubali, A. Marrocchi, A. Broggi, G. Pizzoli, A. Damiani,
Sustainability
2014
,
6
, 6830.
PS1 29
-185-